Precision Medicine Is Transforming Cancer Care.
Traditional medicine relied on symptoms. Precision medicine uses genomic data to diagnose disease and match therapies to the unique molecular profile of each patient’s tumor. Liquid biopsies now enable early detection and real-time monitoring from a simple blood draw—offering faster, less invasive, and scalable solutions.
But challenges remain. Patients with identical tumor mutations often respond differently to the same treatment. Many show no response, even when therapies are guided by detailed tumor data.
The Missing Piece? The Immune System.
The immune system plays a critical role in cancer surveillance and treatment response. Its dynamic interaction with tumors and therapies determines outcomes. Yet, current tools struggle to measure this complexity at scale—limiting patient stratification and slowing drug development.
Unlock Immune Intelligence. At Scale.
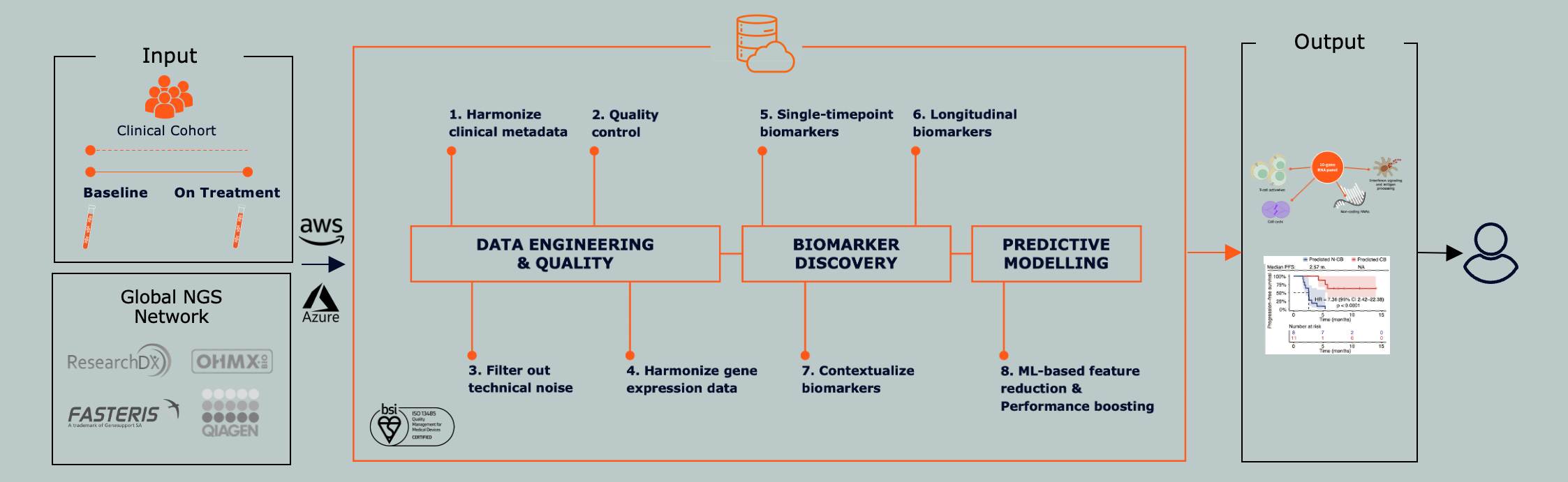
To fully realize precision oncology, we must decode the immune response. Novigenix AI has developed LITOseek™, the first immune liquid biopsy platform based on immuno-transcriptomics.
LITOseek captures gene activity across millions of immune cells in blood, delivering a detailed view of immune dynamics. Our AI integrates this with tumor DNA and multi-omic data to predict response, monitor progress, and improve outcomes.
The platform enables robust immune biomarker discovery and predictive modeling—supporting clinical development, response assessment, and cohort stratification in precision oncology.

Translating Immune Signals into Actionable Insights
Precise and Predictive Data
LITOseek is the first ISO 13485-certified immuno-transcriptomics liquid biopsy platform for longitudinal monitoring of patient response. The multi-omics enables:
- ImmunoPD Response Monitoring
- Mechanism-of-action and resistance insights
- Therapy response prediction
- Toxicity biomarker identification
Advanced Immune Profiling
Understanding the immune system’s role in cancer is key to improving patient outcomes and therapy development. LITOseek reveals the immune response at scale—driving more effective treatments, better patient stratification, and a clearer path toward durable cancer control.
How We do it